The Molecular Structure of Water
Water is an extraordinary compound, primarily due to its unique molecular structure, which results in a variety of special properties. One of the most fascinating characteristics of water is its ability to expand upon freezing. At the fundamental level, this behavior can be attributed to the molecular structure of water. Each water molecule is composed of two hydrogen atoms covalently bonded to one oxygen atom. These atoms are arranged in a bent triangular shape, often represented by the chemical formula H2O. This seemingly simple molecular structure is at the heart of water’s extraordinary characteristics.
The Role of Hydrogen Bonds
The unique properties of water are largely a result of the hydrogen bonds that form between its molecules. These bonds are relatively weak compared to covalent bonds, but they are crucial in defining water’s characteristics. The formation of hydrogen bonds occurs because the oxygen atom in water is more electronegative compared to the hydrogen atoms. This difference in electronegativity causes the water molecule to become polar, with a partial negative charge localized near the oxygen atom and partial positive charges near the hydrogen atoms. The interaction of these partial charges leads to the attraction between different water molecules, forming hydrogen bonds. These bonds give rise to water’s high cohesion and surface tension, which are responsible for phenomena such as water droplets forming as well as its ability to travel upward through plant stems against gravity.
The Impact of Temperature on Water Molecules
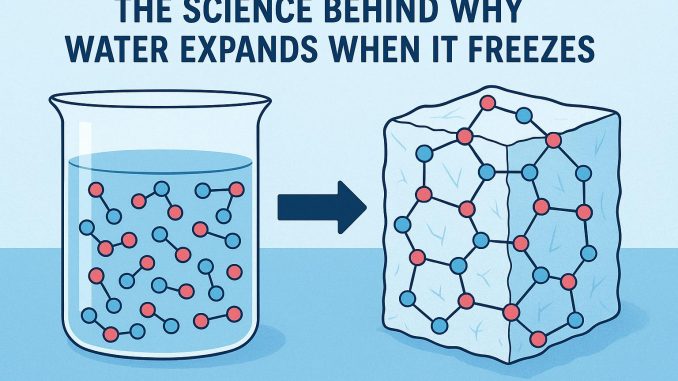
Temperature plays a significant role in affecting the behavior of water molecules. As the temperature of water decreases, the motion of its molecules slows down, allowing more stable hydrogen bonds to form between adjacent water molecules. When water reaches a temperature of about 4°C, it achieves its maximum density. At this point, the molecules are closely packed, resulting in the highest density. However, as the temperature further decreases and approaches 0°C, a remarkable transformation occurs. The hydrogen bonds undergo adjustment, leading to a network where the water molecules arrange themselves in a precise hexagonal lattice, characteristic of ice.
This lattice structure is less dense than that of liquid water due to the molecules being positioned at fixed, slightly greater distances from each other. Consequently, ice floats on liquid water because of this decrease in density. The open lattice arrangement in the solid phase contributes to the anomalous expansion of water upon freezing, where an increase in volume is observed rather than contraction.
The Anomalous Expansion of Water
While the majority of substances contract when transitioning from a liquid to a solid state, water behaves in an anomalous manner due to these hexagonal lattice structures formed by hydrogen bonds. This causes an expansion upon freezing, increasing the volume of solid water compared to its liquid form. As the density decreases, ice becomes buoyant, and thus, it floats on the surface of liquid water. This expansion behavior plays a critical role in environmental dynamics. For instance, in aquatic ecosystems, the floating ice layer acts as an insulating barrier, protecting the aquatic life below from extreme cold. This natural insulation mechanism allows organisms to survive harsh winters in cold climates.
Applications and Implications of Water’s Expansion
Understanding the molecular basis of water’s expansion upon freezing opens numerous avenues for practical applications and implications across various fields. In the realm of engineering and architecture, considerations regarding water’s expansion are vital. For instance, when water pipes freeze during cold weather, the expansion can lead to rupturing, causing significant damage and necessitating repairs. Therefore, it is essential to integrate designs that accommodate this expansion to prevent structural failure.
In environmental science, the expansion behavior of water is crucial for understanding various natural phenomena. Glacier movements, for example, are influenced by the freeze-thaw cycles, which contribute to the carving and shaping of landscapes over time. Similarly, in permafrost regions, the alternation of freezing and thawing affects the integrity of the ground, impacting both natural ecosystems and human-built structures. Additionally, in climate studies, water’s properties are fundamental in predicting and modeling environmental changes, as the melting of polar ice caps and glaciers due to global warming has profound implications on sea levels and climate patterns.
The study of water and its properties also extends into fields such as biology and chemistry, where understanding molecular interactions and behaviors can lead to advancements in fields like biochemistry, pharmacology, and material science. Knowing how water behaves at molecular levels aids in comprehending broader biological processes and chemical reactions, further highlighting the intrinsic value of water’s molecular structure in scientific research and innovation.
Learn more about the properties of water and its wider impact on the environment.
